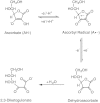
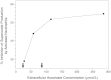
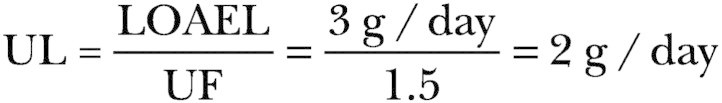-
Afroz M, Bhothinard B, Etzkorn JR, Horenstein S, McGarry JD. 1975. Vitamins C and B12 . J Am Med Assoc 232:246. [PubMed: 1173112]
-
Alexy U, Kersting M, Sichert-Hellert W, Manz F, Schöch G. 1999. Vitamin intake of 3- to 36-month-old German infants and children—Results of the DONALD study. Int J Vitam Nutr Res 69:285–291. [PubMed: 10450535]
-
Allen JC, Keller RP, Archer P, Neville MC. 1991. Studies in human lactation: Milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr 54:69–80. [PubMed: 2058590]
-
Alvares O. 1997. Ascorbic acid and periodontal disease. In: Packer L, Fuchs J, eds. Vitamin C in Health and Disease . New York: Marcel Dekker. Pp.505–516.
-
Anderson D, Phillips BJ, Yu T, Edwards AJ, Ayesh R, Butterworth KR. 1997. The effects of vitamin C supplementation on biomarkers of oxygen radical generated damage in human volunteers with "low" or "high" cholesterol levels. Environ Mol Mutagen 30:161–174. [PubMed: 9329641]
-
Anderson DM, Pittard WB. 1985. Vitamin E and C concentrations in human milk with maternal megadosing. A case report. J Am Diet Assoc 85:715–717. [PubMed: 3998344]
-
Anderson R, Lukey, PT. 1987. A biological role for ascorbate in the selective neutralization of extracellular phagocyte-derived oxidants. Ann NY Acad Sci 498:229–247. [PubMed: 3304062]
-
Anderson R, Oosthuizen R, Maritz R, Theron A, Van Rensburg AJ. 1980. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune function in normal volunteers. Am J Clin Nutr 33:71–76. [PubMed: 7355784]
-
Asami S, Manabe H, Miyake J, Tsurudome Y, Hirano T, Yamaguchi R, Itoh H, Kasai H. 1997. Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site in the human lung. Carcinogenesis 18:1763–1766. [PubMed: 9328173]
-
Auer BL, Auer D, Rodgers AL. 1998. Relative hyperoxaluria, crystalluria and haematuria after megadose ingestion of vitamin C. Eur J Clin Invest 28:695–700. [PubMed: 9767367]
-
Bacon BR, Olynyk JK, Brunt EM, Britton RS, Wolff RK. 1999. HFE genotype in patients with hemochromatosis and other liver diseases. Ann Int Med 130:953–962. [PubMed: 10383365]
-
Baer DM, Tekawa IS, Hurley LB. 1994. Iron stores are not associated with acute myocardial infarction. Circulation 89:2915–2918. [PubMed: 8205708]
-
Baker EM, Sauberlich HE, Wolfskill SJ, Wallace WT, Dean EE. 1962. Tracer studies of vitamin C utilization in men: Metabolism of D -glucuronolactone-6-C14, D -glucuronic-6-C14 acid and L -ascorbic-1-C14 acid . Proc Soc Exp Biol Med 109:737–741.
-
Baker EM, Hodges RE, Hood J, Sauberlich HE, March SC. 1969. Metabolism of ascorbic-1-C14 acid in experimental human scurvy . Am J Clin Nutr 22:549–558. [PubMed: 4891320]
-
Baker EM, Hodges RE, Hood J, Sauberlich HE, March SC, Canham JE. 1971. Metabolism of 14C- and 3H-labeled L -ascorbic acid in human scurvy. Am J Clin Nutr 24:444–454. [PubMed: 5090632]
-
Ballin A, Brown EJ, Koren G, Zipursky A. 1988. Vitamin C-induced erythrocyte damage in premature infants. J Pediatr 113:114–120. [PubMed: 3385519]
-
Bandera EV, Freudenheim JL, Marshall JR, Zielezny M, Priore RL, Brasure J, Baptiste M, Graham S. 1997. Diet and alcohol consumption and lung cancer risk in the New York State Cohort. Cancer Causes Control 8:828–840. [PubMed: 9427425]
-
Barrett B, Gunter E, Jenkins J, Wang M. 1991. Ascorbic acid concentration in amniotic fluid in late pregnancy. Biol Neonate 60:333–335. [PubMed: 1790258]
-
Bates CJ, Prentice AM, Prentice A, Paul AA, Whitehead RG. 1982. Seasonal variations in ascorbic acid status and breast milk ascorbic acid levels in rural Gambian women in relation to dietary intake. Trans Royal Soc Trop Med Hyg 76:341–347. [PubMed: 7112656]
-
Belcher JD, Balla J, Balla G, Jacobs DR Jr, Gross M, Jacob HS, Vercellotti GM. 1993. Vitamin E, LDL, and endothelium. Brief oral vitamin supplementation prevents oxidized LDL-mediated vascular injury in vitro. Arterioscler Thromb 13:1779–1789. [PubMed: 8241098]
-
Bendich A, Cohen M. 1990. Ascorbic acid safety: Analysis of factors affecting iron absorption. Toxicol Lett 51:189–201. [PubMed: 2184546]
-
Berger L, Gerson CD, Yu TF. 1977. The effect of ascorbic acid on uric acid excretion with a commentary on the renal handling of ascorbic acid. Am J Med 62:71–76. [PubMed: 835593]
-
Berger TM, Polidori MC, Dabbagh A, Evans PJ, Halliwell B, Morrow JD, Roberts LJ II, Frei B. 1997. Antioxidant activity of vitamin C in iron-overloaded human plasma. J Biol Chem 272:15656–15660. [PubMed: 9188455]
-
Berliner JA, Heinecke JW. 1996. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med 20:707–727. [PubMed: 8721615]
-
Bermudez E, Stone K, Carter KM, Pryor WA. 1994. Environmental tobacco smoke is just as damaging to DNA as mainstream smoke. Environ Hlth Perspect 102:870–874. [PMC free article: PMC1567343] [PubMed: 9644196]
-
Beutler E. 1991. Glucose-6-phosphate dehydrogenase deficiency. N Engl J Med 324:169–174. [PubMed: 1984194]
-
Blanchard J. 1991. a. Depletion and repletion kinetics of vitamin C in humans. J Nutr 121:170–176. [PubMed: 1995787]
-
Blanchard J. 1991. b. Effects of gender on vitamin C pharmacokinetics in man. J Am Coll Nutr 10:453–459. [PubMed: 1955621]
-
Blanchard J, Conrad KA, Watson RR, Garry PJ, Crawley JD. 1989. Comparison of plasma, mononuclear and polymorphonuclear leucocyte vitamin C levels in young and elderly women during depletion and supplementation. Eur J Clin Nutr 43:97–106. [PubMed: 2707219]
-
Blanchard J, Conrad KA, Garry PJ. 1990. a. Effects of age and intake on vitamin C disposition in females. Eur J Clin Nutr 44:447–460. [PubMed: 2387280]
-
Blanchard J, Conrad KA, Mead RA, Garry PJ. 1990. b. Vitamin C disposition in young and elderly men. Am J Clin Nutr 51:837–845. [PubMed: 2333842]
-
Blanchard J, Tozer TN, Rowland M. 1997. Pharmacokinetic perspectives on megadoses of ascorbic acid. Am J Clin Nutr 66:1165–1171. [PubMed: 9356534]
-
Block G. 1991. Vitamin C and cancer prevention: The epidemiologic evidence. Am J Clin Nutr 53:270S–282S. [PubMed: 1985398]
-
Blot WJ, Li J-Y, Taylor PR, Guo W, Dawsey S, Wang G-Q, Yang CS, Zheng S-F, Gail M, Li G-Y, Yu Y, Liu B-Q, Tangrea J, Sun Y-H, Liu F, Fraumeni JF Jr, Zhang Y-H, Li B. 1993. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 85:1483–1492. [PubMed: 8360931]
-
Bors W, Michel C, Schikora S. 1995. Interaction of flavonoids with ascorbate and determination of their univalent redox potentials: A pulse radiolysis study. Free Radic Biol Med 19:45–52. [PubMed: 7635358]
-
Bostick RM, Potter JD, McKenzie DR, Sellers TA, Kushi LH, Steinmetz KA, Folsom AR. 1993. Reduced risk of colon cancer with high intake of vitamin E: The Iowa Women's Health Study. Cancer Res 53:4230–4237. [PubMed: 8364919]
-
Britton JR, Pavord ID, Richards KA, Knox AJ, Wisniewski AF, Lewis SA, Tattersfield AE, Weiss ST. 1995. Dietary antioxidant vitamin intake and lung function in the general population. Am J Respir Crit Care Med 151:1383–1387. [PubMed: 7735589]
-
Bucca C, Rolla G, Farina JC. 1992. Effect of vitamin C on transient increase of bronchial responsiveness in conditions affecting the airways. Ann NY Acad Sci 669:175–187. [PubMed: 1444023]
-
Bueno de Mesquita HB, Maisonneuve P, Runia S, Moerman CJ. 1991. Intake of foods and nutrients and cancer of the exocrine pancreas: A population-based case-control study in The Netherlands. Int J Cancer 48:540–549. [PubMed: 1646177]
-
Buettner GR. 1993. The pecking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 300:535–543. [PubMed: 8434935]
-
Buettner GR, Jurkiewicz BA. 1996. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat Res 145:532–541. [PubMed: 8619018]
-
Burr ML, Elwood PC, Hole DJ, Hurley RJ, Hughes RE. 1974. Plasma and leukocyte ascorbic acid levels in the elderly. Am J Clin Nutr 27:144–151. [PubMed: 4204847]
-
Bussey HJ, DeCosse JJ, Deschner EE, Eyers AA, Lesser ML, Morson BC, Ritchie SM, Thomson JP, Wadsworth J. 1982. A randomized trial of ascorbic acid in polyposis coli. Cancer 50:1434–1439. [PubMed: 7049351]
-
Butte NF, Garza C, Smith EO, Nichols BL. 1984. Human milk intake and growth in exclusively breast-fed infants. J Pediatr 104:187–195. [PubMed: 6694010]
-
Byerley LO, Kirksey A. 1985. Effects of different levels of vitamin C intake on the vitamin C concentration in human milk and the vitamin C intakes of breastfed infants. Am J Clin Nutr 41:665–671. [PubMed: 3984919]
-
Byun J, Mueller DM, Fabjan JS, Heinecke JW. 1999. Nitrogen dioxide radical generated by the myeloperoxidase-hydrogen peroxide-nitrite system promotes lipid peroxidation of low density lipoprotein. FEBS Lett 455:243–246. [PubMed: 10437781]
-
Cadenas S, Rojas C, Méndez J, Herrero A, Barja G. 1996. Vitamin E decreases urine lipid peroxidation products in young healthy human volunteers under normal conditions. Pharmacol Toxicol 79:247–253. [PubMed: 8936558]
-
Cahill RJ, O'Sullivan KR, Mathias PM, Beattie S, Hamilton H, O'Morain C. 1993. Effects of vitamin antioxidant supplementation on cell kinetics of patients with adenomatous polyps. Gut 34:963–967. [PMC free article: PMC1374235] [PubMed: 8344584]
-
Cameron E, Campbell A. 1974. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact 9:285–315. [PubMed: 4430016]
-
Campbell GD Jr, Steinberg MH, Bower JD. 1975. Ascorbic acid-induced hemolysis in G-6-PD deficiency. Ann Intern Med 82:810. [PubMed: 1138591]
-
Carr AC, Frei B. 1999. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 69:1086–1087. [PubMed: 10357726]
-
Casanueva E, Polo E, Tejero E, Meza C. 1993. Premature rupture of amniotic membranes as functional assessment of vitamin C status during pregnancy. Ann NY Acad Sci 678:369–370. [PubMed: 8494290]
-
Chalmers TC. 1975. Effects of ascorbic acid on the common cold. An evaluation of the evidence. Am J Med 58:532–536. [PubMed: 1092164]
-
Chazan JA, Mistilis SP. 1963. The pathophysiology of scurvy. Am J Med 34:350–358. [PubMed: 14041584]
-
Cheng L, Cohen M, Bhagavan HN. 1985. Vitamin C and the elderly. In: Watson RR, ed. CRC Handbook of Nutrition in the Aged . Boca Raton, FL: CRC Press. Pp.157–185.
-
Choi JL, Rose RC. 1989. Transport and metabolism of ascorbic acid in human placenta. Am J Physiol 257:C110–C113. [PubMed: 2750883]
-
Cohen HA, Neuman I, Nahum H. 1997. Blocking effect of vitamin C in exercise-induced asthma. Arch Pediatr Adolesc Med 151:367–370. [PubMed: 9111435]
-
Cook JD, Watson SS, Simpson KM, Lipschitz DA, Skikne BS. 1984. The effect of high ascorbic acid supplementation on body iron stores. Blood 64:721–726. [PubMed: 6466873]
-
Cooke MS, Evans MD, Podmore ID, Herbert KE, Mistry N, Mistry P, Hickenbotham PT, Hussieni A, Griffiths HR, Lunec J. 1998. Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Lett 439:363–367. [PubMed: 9845354]
-
Coulehan JL, Eberhard S, Kapner L, Taylor F, Rogers K, Garry P. 1976. Vitamin C and acute illness in Navajo school children. N Engl J Med 295:973–977. [PubMed: 787788]
-
Cross CE, Halliwell B. 1993. Nutrition and human disease: How much extra vitamin C might smokers need? Lancet 341:1091. [PubMed: 8096979]
-
Cross CE, Forte T, Stocker R, Louie S, Yamamoto Y, Ames BN, Frei B. 1990. Oxidative stress and abnormal cholesterol metabolism in patients with adult respiratory distress syndrome. J Lab Clin Med 115:396–404. [PubMed: 2324609]
-
Crott JW, Fenech M. 1999. Effect of vitamin C supplementation on chromosome damage, apoptosis and necrosis ex vivo. Carcinogenesis 20:1035–1041. [PubMed: 10357785]
-
Curhan GC, Willett WC, Rimm EB, Stampfer MJ. 1996. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J Urol 155:1847–1851. [PubMed: 8618271]
-
Curhan GC, Willett WC, Speizer FE, Stampfer MJ. 1999. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol 10:840–845. [PubMed: 10203369]
-
Dabrowski K. 1990. Gastro-intestinal circulation of ascorbic acid. Comp Biochem Physiol 95A:481–486.
-
Dallongeville J, Marécaux N, Fruchart J-C, Amouyel P. 1998. Cigarette smoking is associated with unhealthy patterns of nutrient intake: A meta-analysis. J Nutr 128:1450–1457. [PubMed: 9732304]
-
Davies HE, Davies JE, Hughes RE, Jones E. 1984. Studies on the absorption of L xyloascorbic acid (vitamin C) in young and elderly subjects. Hum Nutr Clin Nutr 38C:463–471. [PubMed: 6542908]
-
Davies HE, Gruffudd S, Hughes RE, Jones E. 1987. Ascorbic acid and carnitine in man. Nutr Report Int 36:941–948.
-
DeCosse JJ, Adams MB, Kuzma JF, LoGerfo P, Condon RE. 1975. Effect of ascorbic acid on rectal polyps of patients with familial polyposis. Surgery 78:608–612. [PubMed: 1188603]
-
Delafuente JC, Prendergast JM, Modigh A. 1986. Immunologic modulation by vitamin C in the elderly. Int J Immunopharmacol 8:205–211. [PubMed: 3710663]
-
Delamere NA. 1996. Ascorbic acid and the eye. Subcell Biochem 25:313–329. [PubMed: 8821981]
-
Devaraj S, Jialal I. 1996. Oxidized low-density lipoprotein and atherosclerosis. Int J Clin Lab Res 26:178–184. [PubMed: 8905449]
-
Dewey KG, Finley DA, Lonnerdal B. 1984. Breast milk volume and composition during late lactation (7–20 months). J Pediatr Gastroenterol Nutr 3:713–720. [PubMed: 6502372]
-
Drake IM, Davies MJ, Mapstone NP, Dixon MF, Schorah CJ, White KLM, Chalmers DM, Axon ATR. 1996. Ascorbic acid may protect against human gastric cancer by scavenging mucosal oxygen radicals. Carcinogenesis 17:559–562. [PubMed: 8631145]
-
Duthie SJ, Ma A, Ross MA, Collins AR. 1996. Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer Res 56:1291–1295. [PubMed: 8640816]
-
Dyke GW, Craven JL, Hall R, Garner RC. 1994. a. Effect of vitamin C supplementation on gastric mucosal DNA damage. Carcinogenesis 15:291–295. [PubMed: 8313520]
-
Dyke GW, Craven JL, Hall R, Garner RC. 1994. b. Effect of vitamin C upon gastric mucosal O 6 -alkyltransferase activity and on gastric vitamin C levels. Cancer Lett 86:159–165. [PubMed: 7982203]
-
Eichholzer M, Stahelin HB, Gey KF. 1992. Inverse correlation between essential antioxidants in plasma and subsequent risk to develop cancer, ischemic heart disease and stroke respectively: 12-year follow-up of the Prospective Basel Study. Exp Suppl 62:398–410. [PubMed: 1450600]
-
Ekvall S, Chen IW, Bozian R. 1981. The effect of supplemental ascorbic acid on serum vitamin B12 levels in myelomenigocele patients. Am J Clin Nutr 34:1356–1361. [PubMed: 7258126]
-
Elneihoum AM, Falke P, Hedblad B, Lindgarde F, Ohlsson K. 1997. Leukocyte activation in atherosclerosis: Correlation with risk factors. Atherosclerosis 131:79–84. [PubMed: 9180248]
-
Englard S, Seifter S. 1986. The biochemical functions of ascorbic acid. Annu Rev Nutr 6:365–406. [PubMed: 3015170]
-
Enstrom JE, Kanim LE, Breslow L. 1986. The relationship between vitamin C intake, general health practices, and mortality in Alameda County, California. Am J Pub Hlth 76:1124–1130. [PMC free article: PMC1646565] [PubMed: 3740338]
-
Enstrom JE, Kanim LE, Klein MA. 1992. Vitamin C intake and mortality among a sample of the United States population. Epidemiology 3:194–202. [PubMed: 1591317]
-
Erdman JW Jr, Klein BP. 1982. The influence of harvesting, processing, and cooking on vitamin C in foods. In: Seib PA, Tolbert BM, eds. Ascorbic Acid: Chemistry, Metabolism and Uses . Washington, DC: American Chemical Society. Pp.499–532.
-
Evans RM, Currie L, Campbell A. 1982. The distribution of ascorbic acid between various cellular components of blood in normal individuals, and its relation to the plasma concentration. Br J Nutr 47:473–482. [PubMed: 7082619]
-
FDA (Food and Drug Adminstration). 1985. Nutrient requirements for infant formulas. Fed Regis 50:45106–45108.
-
Fellstrom B, Danielson BG, Karlstrom B, Lithell H, Ljunghall S, Vessby B. 1989. Dietary habits in renal stone patients compared with healthy subjects. Br J Urol 63:575–580. [PubMed: 2752249]
-
Fishbaine B, Butterfield G. 1984. Ascorbic acid status of running and sedentary men. Int J Vitam Nutr Res 54:273. [PubMed: 6500857]
-
Fituri N, Allawi N, Bentley M, Costello J. 1983. Urinary and plasma oxalate during ingestion of pure ascorbic acid: A re-evaluation. Eur Urol 9:312–315. [PubMed: 6628476]
-
Flodin NW. 1988. Pharmacology of Micronutrients . New York: Alan R. Liss. Pp.201–244.
-
Fontham ET. 1994. Vitamin C, vitamin C-rich foods, and cancer: Epidemiologic studies. In: Frei B, ed. Natural Antioxidants in Human Health and Disease . San Diego: Academic Press. Pp.157–197.
-
Fontham ET, Pickle LW, Haenszel W, Correa P, Lin YP, Falk RT. 1988. Dietary vitamins A and C and lung cancer risk in Louisiana. Cancer 62:2267–2273. [PubMed: 3179940]
-
Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. 1991. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci USA 88:11003–11006. [PMC free article: PMC53061] [PubMed: 1763015]
-
Frei B, Stocker R, Ames BN. 1988. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci USA 85:9748–9752. [PMC free article: PMC282858] [PubMed: 3200852]
-
Frei B, England L, Ames BN. 1989. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 86:6377–6381. [PMC free article: PMC297842] [PubMed: 2762330]
-
Frei B, Forte TM, Ames BN, Cross CE. 1991. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochem J 277:133–138. [PMC free article: PMC1151201] [PubMed: 1854329]
-
Freudenheim JL, Graham S, Marshall JR, Haughey BP, Wilkinson G. 1990. A casecontrol study of diet and rectal cancer in western New York. Am J Epidemiol 131:612–624. [PubMed: 2156419]
-
Fuller CJ, Grundy SM, Norkus EP, Jialal I. 1996. Effect of ascorbate supplementation on low density lipoprotein oxidation in smokers. Atherosclerosis 119:139–150. [PubMed: 8808491]
-
Gale CR, Martyn CN, Winter PD, Cooper C. 1995. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. Br Med J 310:1563–1566. [PMC free article: PMC2549941] [PubMed: 7787644]
-
Garry PJ, Goodwin JS, Hunt WC, Gilbert BA. 1982. Nutritional status in a healthy elderly population: Vitamin C. Am J Clin Nutr 36:332–339. [PubMed: 7102589]
-
Garry PJ, Vanderjagt DJ, Hunt WC. 1987. Ascorbic acid intakes and plasma levels in healthy elderly. Ann NY Acad Sci 498:90–99. [PubMed: 3476004]
-
George DR, De Francesca BA. 1989. Human milk in comparison to cow milk. In: Lebenthal E, editor. , ed. Textbook of Gastroenterology and Nutrition in Infancy and Childhood, 2nd edition. New York: Raven Press. Pp.242–243.
-
Gey KF. 1995. Ten-year retrospective on the antioxidant hypothesis of arterioscl rosis: Threshold plasma levels of antioxidant micronutrients related to minimum cardiovascular risk. Nutr Biochem 6:206–236.
-
Gey KF. 1998. Vitamins E plus C and interacting conutrients required for optimal health. A critical and constructive review of epidemiology and supplementation data regarding cardiovascular disease and cancer. Biofactors 7:113–174. [PubMed: 9523035]
-
Gey KF, Stahelin HB, Eichholzer M. 1993. Poor plasma status of carotene and vitamin C is associated with higher mortality from ischemic heart disease and stroke: Basel Prospective Study. Clin Invest 71:3–6. [PubMed: 8453256]
-
Ghadirian P, Boyle P, Simard A, Baillargeon J, Maisonneuve P, Perret C. 1991. Reported family aggregation of pancratic cancer within a population-based case-control study in the francophone community in Montreal, Canada. Int J Pancreatol 10:183–196. [PubMed: 1787333]
-
Giunta JL. 1983. Dental erosion resulting from chewable vitamin C tablets. J Am Dent Assoc 107:253–256. [PubMed: 6578267]
-
Gogel HK, Tandberg D, Strickland RG. 1989. Substances that interfere with guaiac card tests: Implications for gastric aspirate testing. Am J Emerg Med 7:474–480. [PubMed: 2787993]
-
Goldsmith GA. 1961. Human requirements for vitamin C and its use in clinical medicine. Ann NY Acad Sci 92:230–245. [PubMed: 13706612]
-
Gosiewska A, Mahmoodian F, Peterkofsky B. 1996. Gene expression of iron-related proteins during iron deficiency caused by scurvy in guinea pigs. Arch Biochem Biophys 325:295–303. [PubMed: 8561510]
-
Graham S, Zielezny M, Marshall J, Priore R, Freudenheim J, Brasure J, Haughey B, Nasca P, Zdeb M. 1992. Diet in the epidemiology of postmenopausal breast cancer in the New York State Cohort. Am J Epidemiol 136:1327–1337. [PubMed: 1336931]
-
Green MHL, Lowe JE, Waugh APW, Aldridge KE, Cole J, Arlett CF. 1994. Effect of diet and vitamin C on DNA strand breakage in freshly-isolated human white blood cells. Mutat Res 316:91–102. [PubMed: 7521006]
-
Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Beck GJ, Bond JH, Colacchio TA, Coller JA, Frankl HD, Haile RW, Mandel JS, Nierenberg DW, Rothstein R, Snover DC, Stevens MM, Summers RW, van Stolk RU. 1994. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. N Engl J Med 331:141–147. [PubMed: 8008027]
-
Gutteridge JMC. 1991. Plasma ascorbate levels and inhibition of the antioxidant activity of caeruloplasmin. Clin Sci 81:413–417. [PubMed: 1717196]
-
Hallberg L. 1985. The role of vitamin C in improving the critical iron balance situation in women. Int J Vitam Nutr Res 27:177–187. [PubMed: 3926692]
-
Halliwell B. 1998. Can oxidative DNA damage be used as a biomarker of cancer risk in humans? Free Radic Res 29:469–486. [PubMed: 10098453]
-
Halliwell B, Whiteman M. 1997. Antioxidant and prooxidant properties of vitamin C. In: Packer L, editor; , Fuchs J, editor. , eds. Vitamin C in Health and Disease . New York: Marcel Dekker. Pp.59–73.
-
Halliwell B, Wasil M, Grootveld M. 1987. Biologically significant scavenging of the myeloperoxidase-derived oxidant hypochlorous acid by ascorbic acid. FEBS Lett 213:15–17. [PubMed: 3030805]
-
Halpner AD, Handelman GJ, Belmont CA, Harris JM, Blumberg JB. 1998. Protection by vitamin C of oxidant-induced loss of vitamin E in rat hepatocytes. J Nutr Biochem 9:355–359.
-
Hankinson SE, Stampfer MJ, Seddon JM, Colditz GA, Rosner B, Speizer FE, Willett WC. 1992. Nutrient intake and cataract extraction in women: A prospective study. Br Med J 305:335–339. [PMC free article: PMC1882980] [PubMed: 1392884]
-
Harats D, Ben-Naim M, Dabach Y, Hollander G, Havivi E, Stein O, Stein Y. 1990. Effect of vitamin C and E supplementation on susceptibility of plasma lipopr teins to peroxidation induced by acute smoking. Atherosclerosis 85:47–54. [PubMed: 2282108]
-
Harats D, Chevion S, Nahir M, Norman Y, Sagee O, Berry EM. 1998. Citrus fruit supplementation reduces lipoprotein oxidation in young men ingesting a diet high in saturated fat: Presumptive evidence for an interaction between vitamins C and E in vivo. Am J Clin Nutr 67:240–245. [PubMed: 9459371]
-
Harris ED, Percival SS. 1991. A role for ascorbic acid in copper transport. Am J Clin Nutr 54:1193S–1197S. [PubMed: 1962569]
-
Hartz SC, Russell RM, Rosenberg IH. 1992. Nutrition in the Elderly. The Boston Nutr tional Status Survey . London: SmithGordon. P. 38.
-
Haslam RH, Dalby JT, Rademaker AW. 1984. Effects of megavitamin therapy on children with attention deficit disorders. Pediatrics 74:103–111. [PubMed: 6234505]
-
Hatch GE. 1995. Asthma, inhaled oxidants, and dietary antioxidants. Am J Clin Nutr 61:625S–630S. [PubMed: 7879729]
-
Hatch M, Mulgrew S, Bourke E, Keogh B, Costello J. 1980. Effect of megadoses of ascorbic acid on serum and urinary oxalate. Eur Urol 6:166–169. [PubMed: 7371664]
-
Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. 1996. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest 97:1535–1544. [PMC free article: PMC507214] [PubMed: 8617887]
-
Heinecke JW. 1997. Pathways for oxidation of low density lipoprotein by myelope oxidase: Tyrosyl radical, reactive aldehydes, hypochlorous acid and molecular chlorine. BioFactors 6:145–155. [PubMed: 9259996]
-
Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B, Dewey KG. 1993. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: The DARLING Study. Am J Clin Nutr 58:152–161. [PubMed: 8338041]
-
Heitzer T, Just H, Munzel T. 1996. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94:6–9. [PubMed: 8964118]
-
Heller R, Munscher-Paulig F, Grabner R, Till U. 1999. L -Ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J Biol Chem 274:8254–8260. [PubMed: 10075731]
-
Hemila H. 1996. Vitamin C, the placebo effect, and the common cold: A case study of how preconceptions influence the analysis of results. J Clin Epidemiol 49:1079–1084. [PubMed: 8826986]
-
Hemila H. 1997. Vitamin C intake and susceptibility to the common cold. Br J Nutr 77:59–72. [PubMed: 9059230]
-
Hemila H, Herman ZS. 1995. Vitamin C and the common cold: A retrospective analysis of Chalmers' review. J Am Coll Nutr 14:116–123. [PubMed: 7790685]
-
Henning SM, Zhang JZ, McKee RW, Swendseid ME, Jacob RA. 1991. Glutathione blood levels and other oxidant defense indices in men fed diets low in vitamin C. J Nutr 121:1969–1975. [PubMed: 1941261]
-
Herbert V. 1978. Risk of oxalate stones from large doses of vitamin C. N Engl J Med 298:856. [PubMed: 634328]
-
Herbert V. 1995. Vitamin C supplements and disease—Counterpoint. J Am Coll Nutr 14:112–113. [PubMed: 7790683]
-
Herbert V, Jacob E. 1974. Destruction of vitamin B12 by ascorbic acid. J Am Med Assoc 230:241–242. [PubMed: 4479087]
-
Hevia P, Omaye ST, Jacob RA. 1990. Urinary hydroxyproline excretion and vitamin C status in healthy young men. Am J Clin Nutr 51:644–648. [PubMed: 2321570]
-
Hinds MW, Kolonel LN, Hankin JH, Lee J. 1984. Dietary vitamin A, carotene, vitamin C and risk of lung cancer in Hawaii. Am J Epidemiol 119:227–237. [PubMed: 6695902]
-
Hoffer A. 1971. Ascorbic acid and toxicity. N Engl J Med 285:635–636. [PubMed: 5563968]
-
Hoffer A. 1973. Vitamin C and infertility. Lancet 2:1146. [PubMed: 4128030]
-
Hofstad B, Almendingen K, Vatn M, Andersen S, Owen R, Larsen S, Osnes M. 1998. Growth and recurrence of colorectal polyps: A double-blind 3-year intervention with calcium and antioxidants. Digestion 59:148–156. [PubMed: 9586828]
-
Hogenkamp HP. 1980. The interaction between vitamin B12 and vitamin C. Am J Clin Nutr 33:1–3. [PubMed: 7355772]
-
Hornig B, Arakawa N, Kohler C, Drexler H. 1998. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation 97:363–368. [PubMed: 9468210]
-
Hornig D. 1975. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann NY Acad Sci 258:103–118. [PubMed: 1106295]
-
Hornig DH, Moser U. 1981. The safety of high vitamin C intakes in man. In: Counsell JN, editor; , Hornig DH, editor. , eds. Vitamin C (Ascorbic Acid). London: Applied Science. Pp.225–248.
-
Horrobin DF. 1996. Ascorbic acid and prostaglandin synthesis. Subcell Biochem 25:109–115. [PubMed: 8821971]
-
Howe GR, Hirohata T, Hislop TG, Iscovich JM, Yuan JM, Katsouyanni K, Lubin F, Marubini E, Modan B, Rohan T. 1990. Dietary factors and risk of breast cancer: Combined analysis of 12 case-control studies. J Natl Cancer Inst 82:561–. [PubMed: 2156081]
-
Howe GR, Ghadirian P, Bueno de Mesquita HB, Zatonski WA, Baghurst PA, Miller AB, Simard A, Baillargeon J, de Waard F, Przewozniak K. 1992. A collaborative case-control study of nutrient intake and pancreatic cancer within the search programme. Int J Cancer 51:365–372. [PubMed: 1317361]
-
Hoyt CJ. 1980. Diarrhea from vitamin C. J Am Med Assoc 244:1674. [PubMed: 7411820]
-
Hughes C, Dutton S, Truswell AS. 1981. High intakes of ascorbic acid and urinary oxalate. J Hum Nutr 35:274–280. [PubMed: 7276555]
-
Hunt JR, Gallagher SK, Johnson LK. 1994. Effect of ascorbic acid on apparent iron absorption by women with low iron stores. Am J Clin Nutr 59:1381–1385. [PubMed: 8198064]
-
Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE, Willett WC. 1993. A prospective study of the intake of vitamins C, E, and A and the risk of breast cancer. N Engl J Med 329:234–240. [PubMed: 8292129]
-
IOM (Institute of Medicine). 1991. Nutrition During Lactation . Washington, DC: National Academy Press. P. 179. [PubMed: 25144080]
-
Itoh R, Yamada K, Oka J, Echizen H, Murakami K. 1989. Sex as a factor in levels of serum ascorbic acid in a healthy elderly population. Int J Vitam Nutr Res 59:365–372. [PubMed: 2634043]
-
Jacob RA. 1995. The integrated antioxidant system. Nutr Res 15:755–766.
-
Jacob RA. 1999. Vitamin C. In: Shils ME, editor; , Olson JA, editor; , Shike M, editor; , Ross AC, editor. , eds. Modern Nutrition in Health and Disease, 9th edition. Baltimore, MD: Williams & Wilkins. Pp.467–483.
-
Jacob RA, Pianalto FS. 1997. Urinary carnitine excretion increases during experimental vitamin C depletion of healthy men. J Nutr Biochem 8:265–269.
-
Jacob RA, Skala JH, Omaye ST. 1987. a. Biochemical indices of human vitamin C status. Am J Clin Nutr 46:818–826. [PubMed: 3673928]
-
Jacob RA, Skala JH, Omaye ST, Turnlund JR. 1987. b. Effect of varying ascorbic acid intakes on copper absorption and ceruloplasmin levels of young men. J Nutr 117:2109–2115. [PubMed: 3694287]
-
Jacob RA, Otradovec CL, Russell RM, Munro HN, Hartz SC, McGandy RB, Morrow FD, Sadowski JA. 1988. Vitamin C status and nutrient interactions in a healthy elderly population. Am J Clin Nutr 48:1436–1442. [PubMed: 3202092]
-
Jacob RA, Kelley DS, Pianalto FS, Swendseid ME, Henning SM, Zhang Jz, Ames BN, Fraga CG, Peters JH. 1991. Immunocompetence and oxidant defense during ascorbate depletion of healthy men. Am J Clin Nutr 54:1302S–1309S. [PubMed: 1962587]
-
Jacob RA, Pianalto FS, Agee RE. 1992. Cellular ascorbate depletion in healthy men. J Nutr 122:1111–1118. [PubMed: 1564563]
-
Jacob RA, Kutnink MA, Csallany AS, Daroszewska M, Burton GW. 1996. Vitamin C nutriture has little short-term effect on vitamin E concentrations in healthy women. J Nutr 126:2268–2277. [PubMed: 8814216]
-
Jacques PF, Chylack LT Jr. 1991. Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am J Clin Nutr 53:352S–355S. [PubMed: 1985409]
-
Jacques PF, Taylor A, Hankinson SE, Willett WC, Mahnken B, Lee Y, Vaid K, Lahav M. 1997. Long-term vitamin C supplement use and prevalence of early age related lens opacities. Am J Clin Nutr 66:911–916. [PubMed: 9322567]
-
Jaffe RM, Kasten B, Young DS, MacLowry JD. 1975. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med 83:824–826. [PubMed: 1200528]
-
Jama JW, Launer LJ, Witteman JC, den Breeijen JH, Breteler MM, Grobbee DE, Hofman A. 1996. Dietary antioxidants and cognitive function in a population based sample of older persons. The Rotterdam Study. Am J Epidemiol 144:275–280. [PubMed: 8686696]
-
Jariwalla RJ, Harakeh S. 1996. Antiviral and immunomodulatory activities of asco bic acid. Subcell Biochem 25:213–231. [PubMed: 8821976]
-
Jarvinen R, Knekt P, Seppanen R, Teppo L. 1997. Diet and breast cancer risk in a cohort of Finnish women. Cancer Lett 114:251–253. [PubMed: 9103304]
-
Jendryczko A, Tomala J. 1995. The total free radical trapping ability of blood pla ma in eclampsia. Zentralbl Gynakol 117:126–129. [PubMed: 7740845]
-
Jha P, Flather M, Lonn E, Farkouh M, Yusuf S. 1995. The antioxidant vitamins and cardiovascular disease. A critical review of epidemiologic and clinical trial data. Ann Intern Med 123:860–872. [PubMed: 7486470]
-
Jialal I, Devaraj S. 1996. The role of oxidized low density lipoprotein in atherogenesis. J Nutr 126:1053S–1057S. [PubMed: 8642431]
-
Jialal I, Grundy SM. 1991. Preservation of the endogenous antioxidants in low density lipoprotein by ascorbate but not probucol during oxidative modification. J Clin Invest 87:597–601. [PMC free article: PMC296348] [PubMed: 1991843]
-
Jialal I, Vega GL, Grundy SM. 1990. Physiologic levels of ascorbate inhibit the oxidative modification of low density lipoprotein. Atherosclerosis 82:185–191. [PubMed: 2375783]
-
Johnston CS. 1991. Complement component Clq unaltered by ascorbate supplementation in healthy men and women. J Nutr Biochem 2:499–501.
-
Johnston CS. 1999. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 57:71–77. [PubMed: 10101920]
-
Johnston CS, Luo B. 1994. Comparison of the absorption and excretion of three commercially available sources of vitamin C. J Am Diet Assoc 94:779–781. [PubMed: 8021423]
-
Johnston CS, Thompson LL. 1998. Vitamin C status of an outpatient population. J Am Coll Nutr 17:366–370. [PubMed: 9710847]
-
Johnston CS, Martin LJ, Cai X. 1992. Antihistamine effect of supplemental ascorbic acid and neutrophil chemotaxis. J Am Coll Nutr 11:172–176. [PubMed: 1578094]
-
Johnston CS, Meyer CG, Srilakshmi JC. 1993. Vitamin C elevates red blood cell glutathione in healthy adults. Am J Clin Nutr 58:103–105. [PubMed: 8317379]
-
Johnston CS, Solomon E, Corte C. 1996. Vitamin C depletion is associated with alterations in blood histamine and plasma free carnitine in adults. J Am Coll Nutr 15:586–591. [PubMed: 8951736]
-
Kallner A, Hartmann D, Hornig D. 1979. Steady-state turnover and body pool of ascorbic acid in man. Am J Clin Nutr 32:530–539. [PubMed: 420145]
-
Kallner AB, Hartmann D, Hornig DH. 1981. On the requirements of ascorbic acid in man: Steady-state turnover and body pool in smokers. Am J Clin Nutr 34:1347–1355. [PubMed: 7258125]
-
Karra MV, Udipi SA, Kirksey A, Roepke JL. 1986. Changes in specific nutrients in breast milk during extended lactation. Am J Clin Nutr 43:495–503. [PubMed: 3962902]
-
Katsuki H. 1996. Vitamin C and nervous tissue: In vivo and in vitro aspects. Subcell Biochem 25:293–311. [PubMed: 8821980]
-
Keith RE. 1994. Vitamins and physical activity. In: Wolinsky I, editor; , Hickson JF, editor. , eds. Nutrition in Exercise and Sport, 2nd edition. Boca Raton, FL: CRC Press. Pp.159–183.
-
Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. 1999. Altered lung antioxidant status in patients with mild asthma. Lancet 354:482–483. [PubMed: 10465176]
-
Kennes B, Dumont I, Brohee D, Hubert C, Neve P. 1983. Effect of vitamin C supplements on cell-mediated immunity in old people. Gerontology 29:305–310. [PubMed: 6604680]
-
Knekt P, Jarvinen R, Seppanen R, Rissanen A, Aromaa A, Heinonen OP, Albanes D, Heinonen M, Pukkala E, Teppo L. 1991. Dietary antioxidants and the risk of lung cancer. Am J Epidemiol 134:471–479. [PubMed: 1897503]
-
Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. 1994. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol 139:1180–1189. [PubMed: 8209876]
-
Kritchevsky SB, Shimakawa T, Tell G, Dennis B, Carpenter M, Eckfeldt JH, Peacher-Ryan H, Heiss G. 1995. Dietary antioxidants and carotid artery wall thickness. The ARIC Study. Circulation 92:2142–2150. [PubMed: 7554194]
-
Kushi LH, Fee RM, Sellers TA, Zheng W, Folsom AR. 1996. a. Intake of vitamins A, C, and E and postmenopausal breast cancer. The Iowa Women's Health Study. Am J Epidemiol 144:165–174. [PubMed: 8678048]
-
Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. 1996. b. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med 334:1156–1162. [PubMed: 8602181]
-
Lamden MP, Chrystowski GA. 1954. Urinary oxalate excretion by man following ascorbic acid ingestion. Proc Soc Exp Biol Med 85:190–192. [PubMed: 13134331]
-
Laudicina DC, Marnett LJ. 1990. Enhancement of hydroperoxide-dependent lipid peroxidation in rat liver microsomes by ascorbic acid. Arch Biochem Biophys 278:73–80. [PubMed: 2108607]
-
Leaf CD, Vecchio AJ, Roe DA, Hotchkiss JH. 1987. Influence of ascorbic acid dose on N-nitrosoproline formation in humans. Carcinogenesis 8:791–795. [PubMed: 3608076]
-
Leggott PJ, Robertson PB, Rothman DL, Murray PA, Jacob RA. 1986. The effect of controlled ascorbic acid depletion and supplementation on periodontal health. J Periodontol 57:480–485. [PubMed: 3462381]
-
Leggott PJ, Robertson PB, Jacob RA, Zambon JJ, Walsh M, Armitage GC. 1991. Effects of ascorbic acid depletion and supplementation on periodontal health and subgingival microflora in humans. J Dent Res 70:1531–1536. [PubMed: 1663525]
-
Lehr HA, Weyrich AS, Saetzler RK, Jurek A, Arfors KE, Zimmerman GA, Prescott SM, McIntyre TM. 1997. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J Clin Invest 99:2358–2364. [PMC free article: PMC508074] [PubMed: 9153277]
-
Le Marchand L, Yoshizawa CN, Kolonel LN, Hankin JH, Goodman MT. 1989. Vegetable consumption and lung cancer risk: A population-based case-control study in Hawaii. J Natl Cancer Inst 81:1158–1164. [PubMed: 2545891]
-
Lenton KJ, Therriault H, Fulop T, Payette H, Wagner JR. 1999. Glutathione and ascorbate are negatively correlated with oxidative DNA damage in human lymphocytes. Carcinogenesis 20:607–613. [PubMed: 10223188]
-
Leske MC, Chylack LT Jr, Wu SY. 1991. The Lens Opacities Case-Control Study. Risk factors for cataract. Arch Ophthalmol 109:244–251. [PubMed: 1993036]
-
Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF Jr, Vita JA. 1996. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 93:1107–1113. [PubMed: 8653830]
-
Levine M, Dhariwal KR, Wang Y, Park JB, Welch RW. 1994. Ascorbic acid in neutrophils. In: Frei B, editor. , ed. Natural Antioxidants in Health and Disease . San Diego: Academic Press. Pp.469–488.
-
Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. 1996. a. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 93:3704–3709. [PMC free article: PMC39676] [PubMed: 8623000]
-
Levine M, Rumsey S, Wang Y, Park J, Kwon O, Xu W, Amano N. 1996. b. Vitamin C.In: Ziegler EE, editor; , Filer LJ Jr, editor. , eds. Present Knowledge in Nutrition, 7th edition. Washington, DC: ILSI Press. Pp.146–159.
-
Levy R, Shriker O, Porath A, Riesenberg K, Schlaeffer F. 1996. Vitamin C for the treatment of recurrent furunculosis in patients with impaired neutrophil functions. J lnfect Dis 173:1502–1505. [PubMed: 8648230]
-
Loft S, Vistisen K, Ewertz M, Tjonneland A, Overvad K, Poulsen HE. 1992. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: Influence of smoking, gender and body mass index. Carcinogenesis 13:2241–2247. [PubMed: 1473230]
-
Losonczy KG, Harris TB, Havlik RJ. 1996. Vitamin E and vitamin C supplement use and risk of all-cause and coronary heart disease mortality in older persons: The Established Populations for Epidemiologic Studies of the Elderly. Am J Clin Nutr 64:190–196. [PubMed: 8694019]
-
Løvstad RA. 1997. A study on ascorbate inhibition of ceruloplasmin ferroxidase activity. BioMetals 10:123–126. [PubMed: 9210294]
-
LSRO/FASEB (Life Sciences Research Office/Federation of American Societies for Experimental Biology). 1989. Nutrition Monitoring in the United States: An Update Report on Nutrition Monitoring . Prepared for the U.S. Department of Agriculture and the U.S. Department of Health and Human Services. DHHS Publication No. (PHS) 89-1255. Washington, DC: U.S. Government Printing Office.
-
Ludvigsson J, Hansson LO, Tibbling G. 1977. Vitamin C as a preventive medicine against common colds in children. Scand J Infect Dis 9:91–98. [PubMed: 897573]
-
Ludvigsson J, Hansson LO, Stendahl O. 1979. The effect of large doses of vitamin C on leukocyte function and some laboratory parameters. Int J Vitam Nutr Res 49:160–165. [PubMed: 381229]
-
Lunec J, Blake DR. 1985. The determination of dehydroascorbic acid and ascorbic acid in the serum and synovial fluid of patients with rheumatoid arthritis. Free Radic Res Commun 1:31–39. [PubMed: 3880014]
-
Lykkesfeldt J, Loft S, Nielsen JB, Poulsen HE. 1997. Ascorbic acid and dehydroascorbic acid as biomarkers of oxidative stress caused by smoking. Am J Clin Nutr 65:959–963. [PubMed: 9094879]
-
Lykkesfeldt J, Christen S, Wallock LM, Change HH, Jacob RA, Ames BN. 2000. Ascorbate is depleted by smoking and repleted by moderate supplementation: A study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr 71:530–536. [PubMed: 10648268]
-
Mangels AR, Block G, Frey CM, Patterson BH, Taylor PR, Norkus EP, Levander OA. 1993. The bioavailability to humans of ascorbic acid from oranges, o ange juice and cooked broccoli is similar to that of synthetic ascorbic acid. J Nutr 123:1054–1061. [PubMed: 8505665]
-
Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, Fontham ETH, Mera R, Miller MJS, Correa P. 1996. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: Effect of antibiotics and antioxidants. Cancer Res 56:3238–3243. [PubMed: 8764115]
-
Marangon K, Herbeth B, Artur Y, Esterbauer H, Siest G. 1997. Low and very low density lipoprotein composition and resistance to copper-induced oxidation are not notably modified in smokers. Clin Chim Acta 265:1–12. [PubMed: 9352124]
-
Marangon K, Herbeth B, Lecomte E, Paul-Dauphin A, Grolier P, Chancerelle Y, Artur Y. 1998. Diet, antioxidant status, and smoking habits in French men. Am J Clin Nutr 67:231–239. [PubMed: 9459370]
-
May JM, Cobb CE, Mendiratta S, Hill KE, Burk RF. 1998. Reduction of the ascorbyl free radical to ascorbate by thioredoxin reductase. J Biol Chem 273:23039–23045. [PubMed: 9722529]
-
May JM, Mendiratta S, Qu ZC, Loggins E. 1999. Vitamin C recycling and function in human monocytic U-937 cells. Free Radic Biol Med 26:1513–1523. [PubMed: 10401617]
-
McKeown-Eyssen G, Holloway C, Jazmaji V, Bright-See E, Dion P, Bruce WR. 1988. A randomized trial of vitamins C and E in the prevention of recurrence of colorectal polyps. Cancer Res 48:4701–4705. [PubMed: 3293777]
-
McLaran CJ, Bett JHN, Nye JA, Halliday JW. 1982. Congestive cardiomyopathy and haemochromatosis—Rapid progression possibly accelerated by excessive ingestion of ascorbic acid. Aust NZ J Med 12:187–188. [PubMed: 6953962]
-
Melethil S, Mason WD, Chang C-J. 1986. Dose-dependent absorption and excretion of vitamin C in humans. Int J Pharmaceut 31:83–89.
-
Mentzer WC, Collier E. 1975. Hydrops fetalis associated with erythrocyte G-6-PD deficiency and maternal ingestion of fava beans and ascorbic acid. J Pediatr 86:565–567. [PubMed: 1127504]
-
Metz J, Hundertmark U, Pevny I. 1980. Vitamin C allergy of the delayed type. Contact Dermatitis 6:172–174. [PubMed: 7389324]
-
Millar J. 1995. The nitric oxide/ascorbate cycle: How neurones may control their own oxygen supply. Med Hypoth 45:21–26. [PubMed: 8524171]
-
Miller JZ, Nance WE, Norton JA, Wolen RL, Griffith RS, Rose RJ. 1977. Therapeutic effect of vitamin C. A co-twin control study. J Am Med Assoc 237:248–251. [PubMed: 318715]
-
Mirvish SS. 1994. Experimental evidence for inhibition of N-nitroso compound formation as a factor in the negative correlation between vitamin C consumption and the incidence of certain cancers. Cancer Res 54:1948S–1951S. [PubMed: 8137317]
-
Mitch WE, Johnson MW, Kirshenbaum JM, Lopez RE. 1981. Effect of large oral doses of ascorbic acid on uric acid excretion by normal subjects. Clin Pharmcol Ther 29:318–321. [PubMed: 7471601]
-
Montalto MB, Benson JD, Martinez GA. 1985. Nutrient intakes of formula-fed infants and infants fed cow's milk. Pediatrics 75:343–351. [PubMed: 3969338]
-
Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ II. 1995. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. N Engl J Med 332:1198–1203. [PubMed: 7700313]
-
Morse EH, Clark RP, Keyser DE, Merrow SB, Bee DE. 1975. Comparison of the nutritional status of pregnant adolescents with adult pregnant women. I. Biochemical findings. Am J Clin Nutr 28:1000–1013. [PubMed: 1163467]
-
Moser U. 1987. Uptake of ascorbic acid by leukocytes. Ann NY Acad Sci 498:200–215. [PubMed: 3475998]
-
Moss AJ, Levy AS, Kim I, Park YK. 1989. Use of Vitamin and Mineral Supplements in the United States: Current Users, Types of Products, and Nutrients . Advance Data, Vital and Health Statistics of the National Center for Health Statistics. Number 174. Hyattsville, MD: National Center for Health Statistics. Pp.1–19.
-
Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Yoshimura M, Ogawa H, Yasue H. 1997. Endothelium-dependent vasodilation in the brachial artery is impaired in smokers: Effect of vitamin C. Am J Physiol 273:H1644–H1650. [PubMed: 9362226]
-
Mudway IS, Krishna MT, Frew AJ, MacLeod D, Sandstrom T, Holgate ST, Kelly FJ. 1999. Compromised concentrations of ascorbate in fluid lining the respiratory tract in human subjects after exposure to ozone. Occup Environ Med 56:473–481. [PMC free article: PMC1757764] [PubMed: 10472319]
-
Mulholland CW, Strain JJ, Trinick TR. 1996. Serum antioxidant potential, and lipoprotein oxidation in female smokers following vitamin C supplementation. Int J Food Sci Nutr 47:227–231. [PubMed: 8735778]
-
Naidoo D, Lux O. 1998. The effect of vitamin C and E supplementation on lipid and urate oxidation products in plasma. Nutr Res 18:953–961.
-
Ness AR, Khaw KT, Bingham S, Day NE. 1996. Vitamin C status and respiratory function. Eur J Clin Nutr 50:573–579. [PubMed: 8880036]
-
Newmark HL, Scheiner MS, Marcus M, Prabhudesai M. 1976. Stability of vitamin B12 in the presence of ascorbic acid. Am J Clin Nutr 29:645–649. [PubMed: 1274888]
-
Newton HM, Schorah CJ, Habibzadeh N, Morgan DB, Hullin RP. 1985. The cause and correction of low blood vitamin C concentrations in the elderly. Am J Clin Nutr 42:656–659. [PubMed: 4050725]
-
NRC (National Research Council). 1989. Recommended Dietary Allowances , 10th edition. Washington, DC: National Academy Press. [PubMed: 25144070]
-
Nyyssonen K, Parviainen MT, Salonen R, Tuomilehto J, Salonen JT. 1997. a. Vitamin C deficiency and risk of myocardial infarction: Prospective population study of men from eastern Finland. Br Med J 314:634–638. [PMC free article: PMC2126082] [PubMed: 9066474]
-
Nyyssonen K, Poulsen HE, Hayn M, Agerbo P, Porkkala-Sarataho E, Kaikkonen J, Salonen R, Salonen JT. 1997. b. Effect of supplementation of smoking men with plain or slow release ascorbic acid on lipoprotein oxidation. Eur J Clin Nutr 51:154–163. [PubMed: 9076405]
-
Ocke MC, Bueno-de-Mesquita HB, Feskens EJ, van Staveren WA, Kromhout D. 1997. Repeated measurements of vegetables, fruits, beta-carotene, and vit mins C and E in relation to lung cancer. Am J Epidemiol 145:358–365. [PubMed: 9054240]
-
Omaye ST, Skala JH, Jacob RA. 1986. Plasma ascorbic acid in adult males: Effects of depletion and supplementation. Am J Clin Nutr 44:257–264. [PubMed: 3728363]
-
Omaye ST, Schaus EE, Kutnink MA, Hawkes WC. 1987. Measurement of vitamin C in blood components by high-performance liquid chromatography. Implication in assessing vitamin C status. Ann NY Acad Sci 498:389–401. [PubMed: 3304068]
-
Ono K. 1986. Secondary hyperoxalemia caused by vitamin C supplementation in regular hemodialysis patients. Clin Nephrol 26:239–243. [PubMed: 3802587]
-
Oreopoulos DG, Lindeman RD, VanderJagt DJ, Tzamaloukas AH, Bhagavan HN, Garry PJ. 1993. Renal excretion of ascorbic acid: Effect of age and sex. J Am Coll Nutr 12:537–542. [PubMed: 8263270]
-
Ortega RM, Lopez-Sobaler AM, Quintas ME, Martinez RM, Andres P. 1998. The influence of smoking on vitamin C status during the third trimester of pregnancy and on vitamin C levels in maternal milk. J Am Coll Nutr 17:379–384. [PubMed: 9710849]
-
Panayiotidis M, Collins AR. 1997. Ex vivo assessment of lymphocyte antioxidant status using the comet assay. Free Rad Res 27:533–537. [PubMed: 9518069]
-
Pandey DK, Shekelle R, Selwyn BJ, Tangney C, Stamler J. 1995. Dietary vitamin C and beta-carotene and risk of death in middle-aged men. The Western Electric Study. Am J Epidemiol 142:1269–1278. [PubMed: 7503047]
-
Panush RS, Delafuente JC, Katz P, Johnson J. 1982. Modulation of certain immunologic responses by vitamin C. III. Potentiation of in vitro and in vivo ly phocyte responses. Int J Vitam Nutr Res Suppl 23:35–47. [PubMed: 6288604]
-
Park JB, Levine M. 1996. Purification, cloning and expression of dehydroascorbic acid-reducing activity from human neutrophils: Identification as glutaredoxin. Biochem J 315:931–938. [PMC free article: PMC1217296] [PubMed: 8645179]
-
Parkkinen J, Vaaranen O, Vahtera E. 1996. Plasma ascorbate protects coagulation factors against photooxidation. Thromb Haemost 75:292–297. [PubMed: 8815579]
-
Pelletier O. 1977. Vitamin C and tobacco. Int J Vitam Nutr Res Suppl 16:147–170.
-
Perrig WJ, Perrig P, Stahelin HB. 1997. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc 45:718–724. [PubMed: 9180666]
-
Peters EM, Goetzsche JM, Grobbelaar B, Noakes TD. 1993. Vitamin C suppleme tation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am J Clin Nutr 57:170–174. [PubMed: 8185726]
-
Pfeffer F, Valdes-Ramos R, Avila-Rosas H, Meza C, Casanueva E. 1996. Iron, zinc and vitamin C nutritional status is not related to weight gain in pregnant women. Nutr Res 16:555–564.
-
Phull PS, Price AB, White KL, Schorah CJ, Jacyna MR. 1999. Gastroduodenal mucosal vitamin-C levels in Helicobacter pylori infection. Scand J Gastroenterol 34:361–366. [PubMed: 10365895]
-
Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. 1996. Exposure of the US population to environmental tobacco smoke: The Third National Health and Nutrition Examination Survey, 1988 to 1991. J Am Med Assoc 275:1233–1240. [PubMed: 8601954]
-
Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. 1998. Vitamin C exhibits pro-oxidant properties. Nature 392:559. [PubMed: 9560150]
-
Pohl H, Reidy JA. 1989. Vitamin C intake influences the bleomycin-induced chr mosome damage assay: Implications for detection of cancer susceptibility and chromosome breakage syndromes. Mutat Res 224:247–252. [PubMed: 2477699]
-
Powers HJ, Loban A, Silvers K, Gibson AT. 1995. Vitamin C at concentrations observed in premature babies inhibits the ferroxidase activity of caeruloplasmin. Free Radic Res 22:57–65. [PubMed: 7889148]
-
Prieme H, Loft S, Nyyssonen K, Salonen JT, Poulsen HE. 1997. No effect of supplementation with vitamin E, ascorbic acid, or coenzyme Q10 on oxidative DNA damage estimated by 8-hydroxy-7,8-dihydro-2'-deoxyguanosine excretion in smokers. Am J Clin Nutr 65:503–507. [PubMed: 9022536]
-
Pryor WA. 1992. Biological effects of cigarette smoke, wood smoke, and the smoke from plastics: The use of electron spin resonance. Free Radic Biol Med 13:659–676. [PubMed: 1334034]
-
Pryor WA. 1997. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Hlth Perspect 105:875–882. [PMC free article: PMC1470037] [PubMed: 9255574]
-
Pryor WA, Prier DG, Church DF. 1983. Electron-spin resonance study of mai stream and sidestream cigarette smoke: Nature of the free radicals in gasphase smoke and in cigarette tar. Environ Hlth Perspect 47:345–355. [PMC free article: PMC1569403] [PubMed: 6297881]
-
Rajalakshmi R, Deodhar AD, Ramarkrishnan CV. 1965. Vitamin C secretion during lactation. Acta Paediatr Scand 54:375–382. [PubMed: 14343448]
-
Rebouche CJ. 1995. Renal handling of carnitine in experimental vitamin C def ciency. Metabolism 44:1639–1643. [PubMed: 8786736]
-
Rees DC, Kelsey H, Richards JDM. 1993. Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. Br Med J 306:841–842. [PMC free article: PMC1677333] [PubMed: 8490379]
-
Rehman A, Collis CS, Yang M, Kelly M, Diplock AT, Halliwell B, Rice-Evans C. 1998. The effects of iron and vitamin C co-supplementation on oxidative da age to DNA in healthy volunteers. Biochem Biophys Res Commun 246:293–298. [PubMed: 9600109]
-
Reilly M, Delanty N, Lawson JA, Fitzgerald GA. 1996. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation 94:19–25. [PubMed: 8964113]
-
Rhead WJ, Schrauzer GN. 1971. Risks of long-term ascorbic acid overdosage. Nutr Rev 29:262–263. [PubMed: 5127162]
-
Rifici VA, Khachadurian AK. 1993. Dietary supplementation with vitamins C and E inhibits in vitro oxidation of lipoproteins. J Am Coll Nutr 12:631–637. [PubMed: 8294717]
-
Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. 1993. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 328:1450–1456. [PubMed: 8479464]
-
Rivers, JM. 1987. Safety of high-level vitamin C ingestion. Ann NY Acad Sci 498:445–454. [PubMed: 3304071]
-
Robertson JM, Donner AP, Trevithick JR. 1989. Vitamin E intake and risk of cataracts in humans. Ann NY Acad Sci 570:372–382. [PubMed: 2629606]
-
Rokitzki L, Hinkel S, Klemp C, Cufi D, Keul J. 1994. Dietary, serum and urine ascorbic acid status in male athletes. Int J Sports Med 15:435–440. [PubMed: 8002125]
-
Rokkas T, Papatheodorou G, Karameris A, Mavrogeorgis A, Kalogeropoulos N, Giannikos N. 1995. Helicobacter pylori infection and gastric juice vitamin C levels. Impact of eradication. Dig Dis Sci 40:615–621. [PubMed: 7895555]
-
Romney SL, Duttagupta C, Basu J, Palan PR, Karp S, Slagle NS, Dwyer A, Wassertheil-Smoller S, Wylie-Rosett J. 1985. Plasma vitamin C and uterine cervical dysplasia. Am J Obstet Gynecol 151:976–980. [PubMed: 3985059]
-
Ronchetti IP, Quaglino D Jr, Bergamini G. 1996. Ascorbic acid and connective tissue. Subcell Biochem 25:249–264. [PubMed: 8821978]
-
Rose RC, Richer SP, Bode AM. 1998. Ocular oxidants and antioxidant protection. Proc Soc Exp Biol Med 217:397–407. [PubMed: 9521086]
-
Rumsey SC, Levine M. 1998. Absorption, transport, and disposition of ascorbic acid in humans. J Nutr Biochem 9:116–130.
-
Russell AL. 1967. Epidemiology of periodontal disease. Int Dent J 17:282–296. [PubMed: 4862723]
-
Sahyoun NR, Jacques PF, Russell RM. 1996. Carotenoids, vitamins C and E, and mortality in an elderly population. Am J Epidemiol 144:501–511. [PubMed: 8781466]
-
Salmenpera L. 1984. Vitamin C nutrition during prolonged lactation: Optimal in infants while marginal in some mothers. Am J Clin Nutr 40:1050–1056. [PubMed: 6496385]
-
Salonen JT, Salonen R, Nyyssonen K, Korpela H. 1992. Iron sufficiency is associated with hypertension and excess risk of myocardial infarction: The Kuopio Ischemic Heart Disease Risk Factor Study (KIHD). Circulation 85:864–876.
-
Samman S, Brown AJ, Beltran C, Singh S. 1997. The effect of ascorbic acid on plasma lipids and oxidisability of LDL in male smokers. Eur J Clin Nutr 51:472–477. [PubMed: 9234031]
-
Sasaki A, Kondo K, Sakamoto Y, Kurata H, Itakura H, Ikeda Y. 1997. Smoking cessation increases the resistance of low-density lipoprotein to oxidation. Atherosclerosis 130:109–111. [PubMed: 9126654]
-
Satarug S, Haswell-Elkins MR, Tsuda M, Mairiang P, Sithithaworn P, Mairiang E, Esumi H, Sukprasert S, Yongvanit P, Elkins DB. 1996. Thiocyanate-independent nitrosation in humans with carcinogenic parasite infection. Carcinogenesis 17:1075–1081. [PubMed: 8640916]
-
Sauberlich HE. 1994. Pharmacology of vitamin C. Annu Rev Nutr 14 371–391. [PubMed: 7946525]
-
Scaccini C, Jialal I. 1994. LDL Modification by activated polymorphonuclear leukocytes: A cellular model of mild oxidative stress. Free Radic Biol Med 16:49–55. [PubMed: 8299996]
-
Schectman G, Byrd JC, Hoffmann R. 1991. Ascorbic acid requirements for smokers: Analysis of a population survey. Am J Clin Nutr 53:1466–1470. [PubMed: 2035475]
-
Schmidt KH, Hagmaier V, Hornig DH, Vuilleumier JP, Rutishauser G. 1981. Urinary oxalate excretion after large intakes of ascorbic acid in man. Am J Clin Nutr 34:305–311. [PubMed: 7211731]
-
Schrauzer GN, Rhead WJ. 1973. Ascorbic acid abuse: Effects on long-term ingestion of excessive amounts on blood levels and urinary excretion. Int J Vitam Nutr Res 43:201–211. [PubMed: 4716569]
-
Schrauzer GN, Ishmael D, Kiefer GW. 1975. Some aspects of current vitamin C usage: Diminished high-altitude resistance following overdosage. Ann NY Acad Sci 258:377–381. [PubMed: 1060408]
-
Schwartz J, Weiss ST. 1994. Relationship between dietary vitamin C intake and pulmonary function in the First National Health and Nutrition Examination Survey (NHANES I). Am J Clin Nutr 59:110–114. [PubMed: 8279390]
-
Schwarz KB, Cox J, Sharma S, Witter F, Clement L, Sehnert SS, Risby TH. 1995. Cigarette smoking is pro-oxidant in pregnant women regardless of antioxidant nutrient intake. J Nutr Environ Med 5:225–234.
-
Sharpe PC, MacAuley D, McCrum EE, Stott G, Evans AE, Mulholland C, Boreham CA, Duly E, Trinick TR. 1994. Ascorbate and exercise in the Northern Ireland population. Int J Vitam Nutr Res 64:277–282. [PubMed: 7883465]
-
Shekelle RB, Lepper M, Liu S, Maliza C, Raynor WJ, Rossof AH. 1981. Dietary vitamin A and risk of cancer in the Western Electric Study. Lancet 2:1185–1190. [PubMed: 6118627]
-
Shilotri PG, Bhat KS. 1977. Effect of mega doses of vitamin C on bactericidal activity of leukocytes. Am J Clin Nutr 30:1077–1081. [PubMed: 327791]
-
Siegel C, Barker B, Kunstadter M. 1982. Conditioned oral scurvy due to megavitamin C withdrawal. J Periodontol 53:453–455. [PubMed: 6956713]
-
Sies H, Stahl W. 1995. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr 62:1315S–1321S. [PubMed: 7495226]
-
Simon JA. 1992. Vitamin C and cardiovascular disease: A review. J Am Coll Nutr 11:107–125. [PubMed: 1578086]
-
Simon JA, Hudes ES, Browner WS. 1998. Serum ascorbic acid and cardiovascular disease prevalence in US adults. Epidemiology 9:316–321. [PubMed: 9583425]
-
Singh RB, Ghosh S, Niaz MA, Singh R, Beegum R, Chibo H, Shoumin Z, Postiglione A. 1995. Dietary intake, plasma levels of antioxidant vitamins, and oxidative stress in relation to coronary artery disease in elderly subjects. Am J Cardiol 76:1233–1238. [PubMed: 7503002]
-
Sinha R, Block G, Taylor PR. 1993. Problems with estimating vitamin C intakes. Am J Clin Nutr 57:547–550. [PubMed: 8460610]
-
Skaper SD, Fabris M, Ferrari V, Carbonare MD, Leon A. 1997. Quercetin protects cutaneous tissue-associated cell types including sensory neurons from oxidative stress induced by glutathione depletion: Cooperative effects of ascorbic acid. Free Radic Biol Med 22:669–678. [PubMed: 9013129]
-
Sneed SM, Zane C, Thomas MR. 1981. The effects of ascorbic acid, vitamin B6, vitamin B12, and folic acid supplementation on the breast milk and maternal nutritional status of low socioeconomic lactating women. Am J Clin Nutr 34:1338–1346. [PubMed: 7258124]
-
Solzbach U, Hornig B, Jeserich M, Just H. 1997. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation 96:1513–1519. [PubMed: 9315540]
-
Specker BL, Beck A, Kalkwarf H., Ho M. 1997. Randomized trial of varying mineral intake on total body bone mineral accretion during the first year of life. Pediatrics 99:e12. [PubMed: 9164808]
-
Stein HB, Hasan A, Fox IH. 1976. Ascorbic acid-induced uricosuria. Ann Intern Med 84:385–388. [PubMed: 1259282]
-
Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. 1998. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 97:2222–2229. [PubMed: 9631871]
-
Thomas MR, Kawamoto J, Sneed SM, Eakin R. 1979. The effects of vitamin C, vitamin B6, and vitamin B12 supplementation on the breast milk and maternal status of well-nourished women. Am J Clin Nutr 32:1679–1685. [PubMed: 463805]
-
Thomas MR, Sneed SM, Wei C, Nail PA, Wilson M, Sprinkle EE. 1980. The effects of vitamin C, vitamin B6, vitamin B12, folic acid, riboflavin, and thiamin on the breast milk and maternal status of well-nourished women at 6 months postpartum. Am J Clin Nutr 33:2151–2156. [PubMed: 7424809]
-
Timimi FK, Ting HH, Haley EA, Roddy MA, Ganz P, Creager MA. 1998. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol 31:552–557. [PubMed: 9502634]
-
Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. 1996. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 97:22–28. [PMC free article: PMC507058] [PubMed: 8550838]
-
Ting HH, Timimi FK, Haley EA, Roddy MA, Ganz P, Creager MA. 1997. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation 95:2617–2622. [PubMed: 9193429]
-
Tiselius HG, Almgard LE. 1977. The diurnal urinary excretion of oxalate and the effect of pyridoxine and ascorbate on oxalate excretion. Eur Urol 3:41–46. [PubMed: 556987]
-
Tlaskal P, Novakova V. 1990. Vitamins C and E in neonates and their mothers. Cesk Pediatr 45:339–343. [PubMed: 2289252]
-
Tribble DL, Giuliano LJ, Fortmann SP. 1993. Reduced plasma ascorbic acid concentrations in nonsmokers regularly exposed to environmental tobacco smoke. Am J Clin Nutr 58:886–890. [PubMed: 8249873]
-
Tsao CS. 1997. An overview of ascorbic acid chemistry and biochemistry. In: Packer L, editor; , Fuchs J, editor. , eds. Vitamin C in Health and Disease . New York: Marcel Dekker. Pp.25–58.
-
Tsao CS, Leung PY. 1988. Urinary ascorbic acid levels following the withdrawal of large doses of ascorbic acid in guinea pigs. J Nutr 118:895–900. [PubMed: 3392599]
-
Tsao CS, Salimi SL. 1984. Effect of large intake of ascorbic acid on urinary and plasma oxalic acid levels. Int J Vitam Nutr Res 54:245–249. [PubMed: 6500850]
-
Udipi SA, Kirksey A, West K, Giacoia G. 1985. Vitamin B6, vitamin C and folacin levels in milk from mothers of term and preterm infants during the neonatal period. Am J Clin Nutr 42:522–530. [PubMed: 4041128]
-
Urivetzky M, Kessaris D, Smith AD. 1992. Ascorbic acid overdosing: A risk factor for calcium oxalate nephrolithiasis. J Urol 147:1215–1218. [PubMed: 1569652]
-
Valkonen M, Kuusi T. 1998. Passive smoking induces atherogenic changes in low-density lipoprotein. Circulation 97:2012–2016. [PubMed: 9610530]
-
VanderJagt DJ, Garry PJ, Bhagavan HN. 1987. Ascorbic acid intake and plasma levels in healthy elderly people. Am J Clin Nutr 46:290–294. [PubMed: 3618533]
-
Van Eekelen M. 1953. Occurrence of vitamin C in foods. Proc Nutr Soc 12:228–232.
-
Vitale S, West S, Hallfrisch J, Alston C, Wang F, Moorman C, Muller D, Singh V, Taylor HR. 1993. Plasma antioxidants and risk of cortical and nuclear cataract. Epidemiology 4:195–203. [PubMed: 8512984]
-
Vogel RI, Lamster IB, Wechsler SA, Macedo B, Hartley LJ, Macedo JA. 1986. The effects of megadoses of ascorbic acid on PMN chemotaxis and experimental gingivitis. J Periodontol 57:472–479. [PubMed: 3462380]
-
Wandzilak TR, D'Andre SD, Davis PA, Williams HE. 1994. Effect of high dose vitamin C on urinary oxalate levels. J Urol 151:834–837. [PubMed: 8126804]
-
Wang Y, Russo TA, Kwon O, Chanock S, Rumsey SC, Levine M. 1997. Ascorbate recycling in human neutrophils: Induction by bacteria. Proc Natl Acad Sci USA 94:13816–13819. [PMC free article: PMC28390] [PubMed: 9391110]
-
Waring AJ, Drake IM, Schorah CJ, White KL, Lynch DA, Axon AT, Dixon MF. 1996. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: Effects of gastritis and oral supplementation. Gut 38:171–176. [PMC free article: PMC1383018] [PubMed: 8801192]
-
Wassertheil-Smoller S, Romney SL, Wylie-Rosett J, Slagle S, Miller G, Lucido D, Duttagupta C, Palan PR. 1981. Dietary vitamin C and uterine cervical dysplasia. Am J Epidemiol 114:714–724. [PubMed: 7304600]
-
Weber C, Wolfgang E, Weber K, Weber PC. 1996. Increased adhesiveness of isolated monocytes to endothelium is prevented by vitamin C intake in smokers. Circulation 93:1488–1492. [PubMed: 8608614]
-
Wen Y, Cooke T Feely, J. 1997. The effect of pharmacological supplementation with vitamin C on low-density lipoprotein oxidation. Br J Clin Pharmacol 44:94–97. [PMC free article: PMC2042803] [PubMed: 9241103]
-
Witt EH, Reznick AZ, Viguie CA, Starke-Reed P, Packer L. 1992. Exercise, oxidative damage and effects of antioxidant manipulation . J Nutr 122:766–773. [PubMed: 1514950]
-
Woolfe SN, Kenney EB, Hume WR, Carranza FA Jr. 1984. Relationship of ascorbic acid levels of blood and gingival tissue with response to periodontal therapy. J Clin Periodontol 11:159–165. [PubMed: 6368610]
-
Yong LC, Brown CC, Schatzkin A, Dresser CM, Slesinski MJ, Cox CS, Taylor PR. 1997. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I Epidemiologic Followup Study. Am J Epidemiol 146:231–243. [PubMed: 9247007]
-
Young JC, Kenyon EM, Calabrese EJ. 1990. Inhibition of beta-glucuronidase in human urine by ascorbic acid. Hum Exp Toxicol 9:165–170. [PubMed: 2375883]
-
Zatonski W, Przewozniak K, Howe GR, Maisonneuve P, Walker AM, Boyle P. 1991. Nutritional factors and pancreatic cancer: A case-control study from south west Poland. Int J Cancer 48:390–394. [PubMed: 2040534]